Throughout the history of industrial chemistry, it is not just molecules that have driven progress — it is the materials that control them. The catalysts and adsorbents we design to guide molecular transformations and separations have consistently catalyzed not only reactions, but entire technological revolutions.
From the emergence of fluid catalytic cracking to the synthesis of ammonia, methanol, sulfuric acid, and countless others, nearly 90% of processes in the chemical and refining industries rely on catalysis. These transformations — essential to fuel production, polymer manufacturing, fertilizers, detergents, and more — have been enabled and continuously improved by advances in solid materials engineering. Catalysis and adsorption technologies are not static: they are dynamic responses to evolving industrial demands — tighter environmental regulations, more complex feedstocks, energy efficiency constraints, and now, decarbonization and circularity.
From Zeolites to Tailored Oxides: A Legacy of Incremental Innovation
During the second half of the 20th century, the petrochemical and refining industries shaped the field of heterogeneous catalysis. Zeolites such as Y and ZSM-5 enabled selective cracking and isomerization under high-temperature conditions, while amorphous silica-alumina and promoted γ-alumina became standard supports for hydroprocessing and hydrogenation reactions. These were further refined with the development of NiMo, CoMo, and NiW sulfided catalysts, optimized for hydrodesulfurization (HDS), hydrodenitrogenation (HDN), and aromatic saturation.
In parallel, dense oxide systems — V₂O₅/TiO₂ for selective catalytic reduction (SCR), Fe/Cr spinels for high-pressure ammonia synthesis, or Pt-based gauzes for nitric acid — provided the thermal and mechanical robustness required for large-scale fixed-bed reactors. Platinum-group metal (PGM) catalysts also enabled key oxidation and hydrogenation reactions under milder conditions in both gas and liquid phases.
The 1980s and 1990s saw the introduction of structured catalysts and monoliths, especially in environmental applications and gas-phase oxidation, where low pressure drop and high geometric surface area were essential. These materials became essential for automotive exhaust treatment, VOC abatement, and compact reformers.
Catalytic reforming and isomerization processes benefited from bifunctional catalysts combining acid and metallic sites — typically Pt or Re on chlorinated alumina or zeolites — enabling both skeletal rearrangement and hydrogenation-dehydrogenation in a single step. Similarly, oxidation catalysts for partial oxidation and steam reforming evolved toward higher dispersion, stronger metal-support interaction, and sulfur resistance.
In adsorption, the 1990s and 2000s brought further advances in high-capacity adsorbents for air purification, solvent recovery, and water treatment. Activated carbons, aluminas, silica gels, and synthetic resins were fine-tuned for mercury, VOCs, acid gases, and moisture removal. Later, functionalized adsorbents — including ion-exchange resins and hybrid organo-mineral materials — opened applications in pharmaceuticals, food processing, and ultrapure gas production.
Despite these advances, traditional materials — though reliable and mature — offer limited control over pore architecture and internal chemical environment. In an era where molecular-level selectivity, multifunctionality, and process intensification are increasingly valued, new materials are needed to overcome these intrinsic limitations
The Rise of MOFs: From Structural Novelty to Functional Opportunity
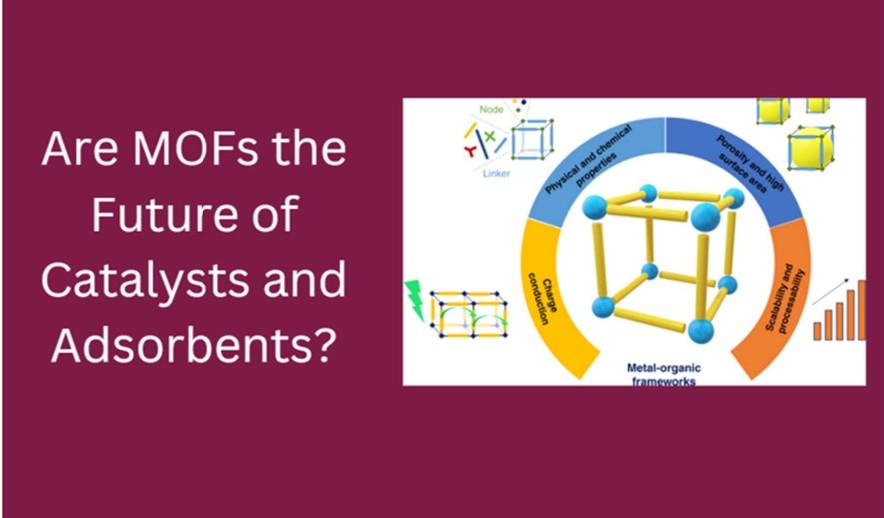
Metal-Organic Frameworks (MOFs) represent a fundamentally different approach. These crystalline, hybrid materials are assembled from inorganic nodes and organic linkers, forming periodic networks with uniform and tunable porosity. Unlike zeolites or carbons, MOFs offer modular design at the molecular level — pore size, geometry, and chemical functionality can be precisely engineered.
This structural versatility enables properties that were previously inaccessible:
– Ultra-high surface areas exceeding 6,000 m²/g
– Pore size control from micropores (<2 nm) to mesopores (>2 nm)
– Chemical functionalization tailored to specific guest molecules
– Multifunctionality, allowing adsorption and catalysis in the same matrix
In adsorption, MOFs have demonstrated remarkable selectivity for gases like CO₂, C₂H₂, NH₃, H₂S, and VOCs — with performance surpassing that of classical materials, especially under dilute or humid conditions. This is particularly relevant for applications in direct air capture, post-combustion CO₂ removal, and gas-phase separations in petrochemical streams.
In catalysis, MOFs serve not only as inert supports but as active, designable platforms. They can incorporate Lewis or Brønsted acid sites, immobilized transition metals, or confined catalytic nanoparticles. Their regular porosity offers a level of site accessibility and product diffusion that can be finely tuned — enabling applications from tandem catalysis to photocatalytic and electrocatalytic systems.
Despite their promise, MOFs faced early skepticism due to concerns about scalability, stability, and cost. However, this landscape is changing rapidly. Thermally and chemically stable families — such as ZIFs, UiOs, MILs, and PCNs — have shown resilience under industrially relevant conditions, including steam, acid/base exposure, and cyclic adsorption-regeneration processes. Meanwhile, advances in solvent-free synthesis, continuous flow reactors, and ligand recycling have reduced production costs and environmental impact.
Several companies and consortia are now producing MOFs at pilot and tonne scale, targeting applications in gas purification, CO₂ capture, catalytic membranes, and structured reactors. In parallel, regulatory frameworks are beginning to accept MOFs for use in air treatment and energy storage devices.
Looking ahead, MOFs are unlikely to replace conventional materials across the board. Instead, they will expand the design space for catalysis and adsorption — particularly in high-value, high-selectivity processes where conventional materials fall short. This includes hybrid systems (e.g., MOF–zeolite composites), structured catalysts, and integrated capture-conversion platforms.
In that sense, MOFs are not just a new class of materials — they represent a new paradigm for engineering molecular function from the ground up.
At MERYT, we are actively investing in this new generation of materials — particularly in applications related to CO₂ capture and the catalytic conversion of captured CO₂ into methanol — because we believe MOFs will play a defining role in the future of sustainable industrial chemistry.