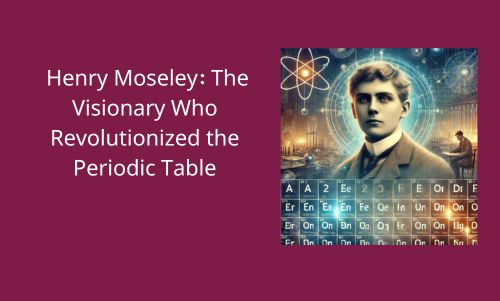
As we approach November 23, we celebrate the birthday of Henry Gwyn Jeffreys Moseley, a brilliant physicist and chemist born in Weymouth, England, in 1887. Moseley grew up in a family dedicated to science; his father, Henry Nottidge Moseley, was a distinguished biologist and professor at Oxford University, and his mother, Amabel Moseley, encouraged his intellectual curiosity. From an early age, Moseley excelled in mathematics and science, eventually attending Eton College and later Trinity College, Oxford, where he graduated with a degree in natural sciences in 1910.
After completing his studies, Moseley joined the University of Manchester, where he worked under the supervision of Ernest Rutherford, one of the most influential physicists of the time. Rutherford, often referred to as the “father of nuclear physics”, is best known for his discovery of the nucleus through the famous gold foil experiment and for developing the planetary model of the atom. Working alongside Rutherford, Moseley was at the forefront of atomic research during one of the most revolutionary periods in science.
Moseley’s most significant achievements came from his pioneering work in X-ray spectroscopy, a technique that allowed him to study the properties of elements. By analyzing the X-rays emitted by various elements, Moseley discovered a direct relationship between the frequency of the emitted radiation and the number of protons in the atom’s nucleus. This breakthrough led to his defining discovery of the atomic number as the unique identifier of each element, transforming the periodic table and how we understand atomic structure.
During his short but impactful career, Moseley’s contributions included:
- Establishing the concept of atomic number: Moseley proved that the number of protons in an atom’s nucleus determines its identity, refining the periodic table’s organization.
- Correcting inconsistencies in the periodic table: His work resolved misplaced elements like iodine and tellurium by basing the arrangement on atomic number rather than atomic weight.
- Predicting missing elements: Moseley identified gaps in the periodic table, paving the way for the discovery of elements such as technetium and hafnium.
- Bridging chemistry and physics: His use of X-ray spectroscopy linked atomic structure to elemental properties, uniting these fields.
Tragically, Moseley’s career and life were cut short when he enlisted in the British Army during World War I. He was killed in action at the Battle of Gallipoli in 1915 at the age of just 27. His untimely death not only highlighted the immense loss of scientific talent caused by war but also spurred changes in military policies to protect scientists from combat roles.
Though his life was brief, Henry Moseley’s legacy remains profound. His discovery of the atomic number as a fundamental property of elements reshaped the periodic table and laid the groundwork for advancements in quantum mechanics, nuclear physics, and chemistry. His collaboration with Rutherford symbolizes a golden era in atomic research, where the foundations of modern science were being built.
On November 23, we honor Henry Moseley not only for his groundbreaking contributions to science but also for his relentless pursuit of knowledge. His story reminds us of the transformative power of curiosity and dedication, inspiring generations of scientists to follow in his footsteps.